Access Denied: NIH’s Data Crackdown Marks a Turning Point in U.S. Scientific and Strategic Policy
In a move that has sent shockwaves across the global scientific community, the National Institutes of Health has abruptly revoked access to its Controlled-Access Data Repositories, including the SEER cancer database, for researchers and institutions based in six countries designated as "countries of concern" by U.S. federal policy. Effective April 4, the decision is part of a broader, aggressive national security initiative aiming to cordon off sensitive biomedical and personal data from geopolitical rivals.
For thousands of researchers worldwide, the announcement landed without warning. SEER*Stat logins quietly failed. Access was cut mid-project. And inquiries were met with a single, curt message: “We are unable to restore access to your account at this time.”
This sudden fracture in scientific collaboration reflects far more than a technical update—it marks a profound recalibration of the balance between global research cooperation and national security imperatives.
A Fortress Around Data: The Policy Behind the Lockout
The NIH’s action follows Executive Order 14117 and newly enforced federal regulations under 28 CFR Part 202, which explicitly prohibit access to sensitive personal and government-related data for entities in China (including Hong Kong and Macau), Russia, Iran, North Korea, Cuba, and Venezuela. This prohibition not only terminates ongoing projects involving the SEER database—a critical cancer research tool—but applies to all NIH Controlled-Access Data Repositories .
This policy was codified in NIH Guide Notice NOT-OD-25-083, but it’s the real-world ripple effect that’s proving to be most disruptive.
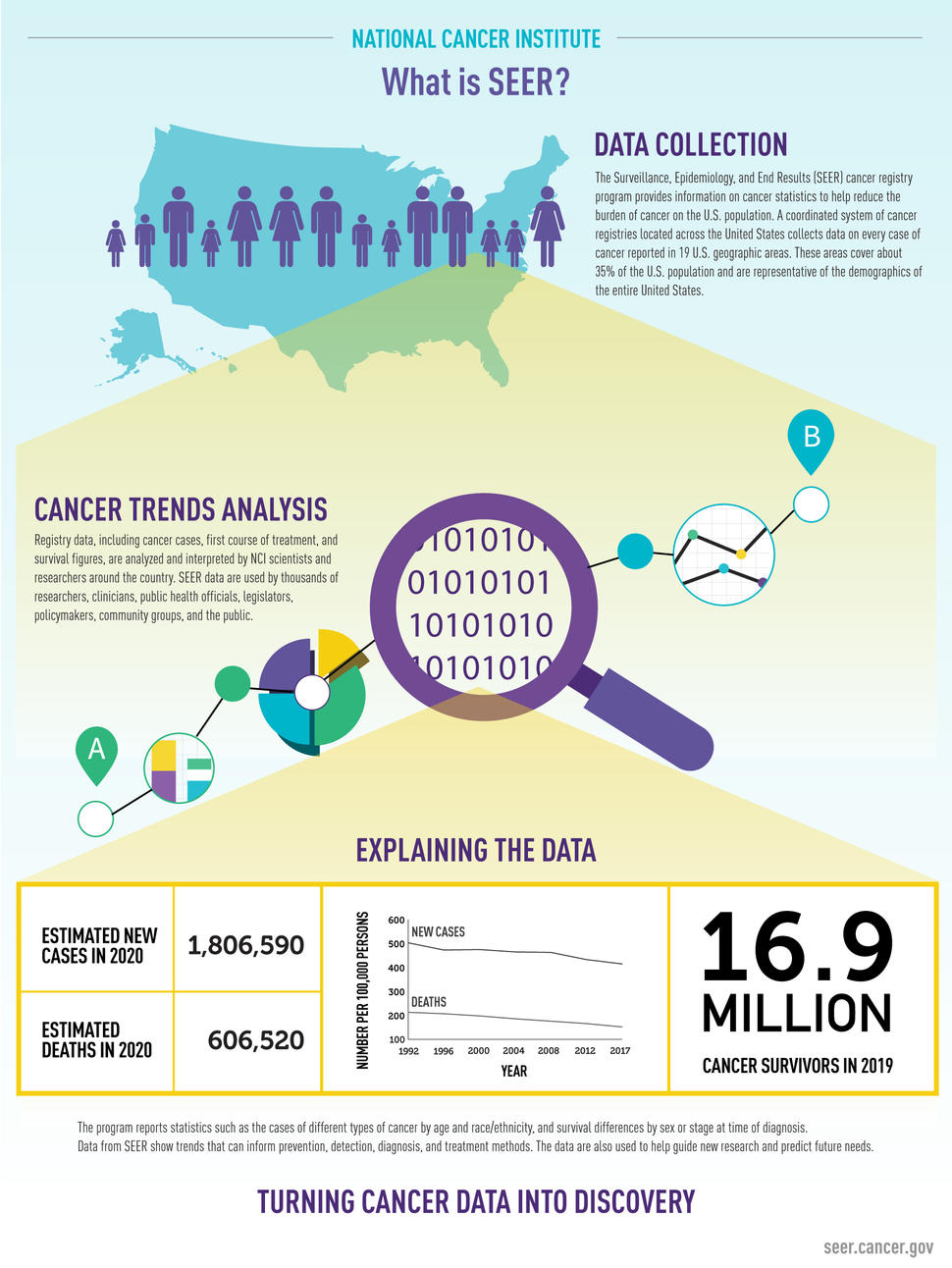
The SEER database is a linchpin of American cancer research. Covering roughly 48% of the U.S. population, it contains decades of meticulously curated data on cancer incidence, treatment, and survival. The database is a vital tool for modeling treatment outcomes, understanding health disparities, and planning national cancer control strategies.
“This is like abruptly shutting off the water main to half the oncology research pipeline,” said one senior U.S.-based epidemiologist, who requested anonymity to speak candidly. “No matter how good your lab is, if you can’t access population-level data, your work becomes guesswork.”
“Necessary Security or Scientific Setback?”: The War of Perspectives
The policy shift has split the research and policy communities, exposing a growing chasm between national security priorities and the traditionally borderless ethos of scientific inquiry.
A Case for Restriction: The National Security Lens
Supporters of the move argue it is long overdue. A cybersecurity analyst with experience advising federal agencies stated, “You wouldn’t hand your adversary a blueprint to your infrastructure. Why should biomedical data be any different?”
Data misuse scenarios—ranging from AI-enabled profiling and predictive surveillance to the weaponization of genetic vulnerabilities—have transformed academic datasets into geopolitical assets. According to a policy memo circulated internally by an NIH advisory group, access to longitudinal U.S. health data could enable foreign intelligence agencies to construct detailed behavioral and biometric profiles of the American population.
“People assume cancer data is benign,” said a consultant who advises tech-sector compliance teams. “But combine it with other datasets—genomics, demographics, location metadata—and suddenly you have a dataset that’s both commercially exploitable and strategically dangerous.”
Scientific Community Pushback: “Politics is Poisoning Progress”
Yet to many in the global health and scientific communities, the NIH’s move appears dangerously overbroad and politically charged.
Critics argue the policy will decimate multi-year, multinational collaborations, particularly in cancer and rare disease research, where sample sizes often depend on cross-border aggregation of cases. The potential for slowed breakthroughs in early diagnosis and treatment modeling is real.
“We’re in an era of precision medicine,” said a public health advocate affiliated with a leading international NGO. “Precision dies without data.”
Beyond practical concerns, the symbolic damage is profound. “This sends a chilling message that science is no longer immune to geopolitics,” one legal scholar commented. “It sets a precedent where where-you’re-from overrides what-you’re-working-on.”
Several civil liberties organizations, including international academic consortiums, have also raised concerns about discrimination and the erosion of open-access research principles long championed by the U.S.
Global Decoupling: A Broader Arc of Isolation
The SEER data ban is not an isolated act—it is part of a sweeping recalibration of U.S. research and academic policy, particularly toward China.
Parallel Actions Reinforce the Wall
-
Tech Sector Restrictions: The U.S. has expanded export controls on critical technologies—AI chips, advanced semiconductors, quantum computing—to block Chinese access.
-
Academic and Visa Policies: Federal proposals aim to restrict or ban student visas for Chinese nationals in STEM fields. Some U.S. universities have preemptively ended joint programs with Chinese institutions.
-
State-Level Actions: States like Texas have mandated severance of ties with Chinese government-linked entities and imposed new vetting protocols for international collaborations.
-
Closure of Confucius Institutes: A majority of Confucius Institutes in the U.S. have shuttered, following federal pressure tying research funding to the absence of these programs.
-
Revised Bilateral Pacts: A reworked science and technology agreement with China, signed under the Biden administration, preserved only the most basic research pathways while embedding strict safeguards on data and IP sharing.
Collectively, these steps form a coherent decoupling strategy—one not only of capital and technology but now of knowledge itself.
Strategic and Financial Implications: What Traders and Investors Need to Watch
For markets and policy analysts, the NIH’s policy reveals more than bureaucratic risk management—it reveals tectonic realignments in the global innovation landscape.
1. Health Data as National Security Asset
Health data is now officially classified, implicitly, as a national strategic asset. For companies in biotech, genomics, and AI diagnostics, this reframing means stricter controls on research partnerships, heightened due diligence for M&A activity, and possible enforcement actions for non-compliance.
Investors should evaluate portfolio companies’ exposure to international research dependencies and their agility in reconfiguring research pipelines.
2. Surge in Domestic R&D Infrastructure
Expect a renewed surge in federal funding to bolster U.S.-based research capacity, particularly in oncology, neuroscience, and pandemic preparedness. Institutions and firms with robust domestic infrastructure stand to benefit—think domestic CROs, academic spinouts, and U.S.-only data platforms.
3. Rise of Competing Data Sovereignties
As China accelerates its own biomedical data programs, the world could see the emergence of dueling epidemiological ecosystems, each jealously guarding data access while racing to develop proprietary health insights. This fragmentation may disadvantage multinational pharma firms that rely on globally harmonized trial data and cross-border AI modeling.
“There will be no one ‘truth’ in health data going forward—just Western data and Eastern data,” said one market research consultant.
4. Volatility from Regulatory Spillovers
The line between “controlled data” and “commercial analytics” is blurring. As the U.S. government tightens definitions of sensitive datasets, adjacent sectors—health informatics, digital therapeutics, wearable health devices—may also face scrutiny.
Anticipate increased regulatory volatility, especially in cross-listed tech-health hybrids and AI healthcare platforms operating across borders.
What Comes Next: A Science Without Borders or a World Without Shared Science?
The NIH's decision may be a prelude to a wider fortress-building strategy, where scientific collaboration is subjected to the same decoupling logic applied to chips and capital.
But the cost of such a pivot is immense.
Cancer doesn’t respect borders. Neither do pandemics. And while adversarial state actors may abuse access, the vast majority of researchers in the affected countries are physicians, epidemiologists, and data scientists—many trained in the U.S.—working to reduce human suffering.
“This is more than a policy change,” one U.S. researcher lamented. “It’s the end of an era.”
A Strategic Firewall with High Human Stakes
In locking down access to its premier biomedical datasets, the United States has drawn a new line in the sand—one that may redefine not just international research but the very nature of scientific progress in a fractured world.
For national security hawks, the move is prudent, even overdue. For the scientific community, it is painful, even perilous. For investors, it signals both disruption and opportunity.
The question is not just who gets to see the data—but what kind of world we build when access is no longer equal.
